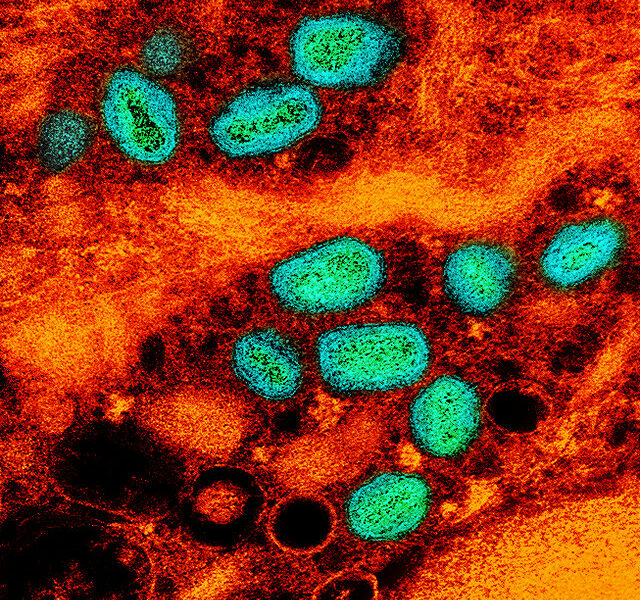
In mid December of 2011, four infants were diagnosed with a rare bacterial infection. Two died. The infecting agent was Cronobacter sakazakii, formerly known as Enterobacter sakazakii. The US Centers for Disease Control and Prevention typically see four to six cases a year; however, in 2011 twelve cases were recorded. These most recent cases were found in four different states: Missouri, Illinois, Oklahoma and Florida.
As a precautionary measure, retailers recalled a powdered infant formula, Mead Johnson’s Enfamil, due to supposed association with the C. sakazakii infections. Two of the infected infants consumed Enfamil brand powdered baby formula, while the other two reportedly drank several different brands.
Tests conducted by the CDC revealed that the C. sakazakii found in Missouri and Illinois are genetically different, suggesting that the cases are not related. While tests from Missouri show presence of the bacteria in an open container of Enfamil as well as in an open container of nursery water (a distilled water used to prepare infant formula), no traces of the bacteria were found in other sealed containers of the powdered formula tested from the same lot. How the water and powder were contaminated remains unclear and the investigation continues.
In a joint statement released Dec. 30, the FDA and the CDC stated that there is no need for a recall of Enfamil formula.
Currently, the CDC is asking that public health officials nationwide be vigilant for other cases of Cronobacter infection among infants.
Symptoms of C. sakazakii infection include fever, poor feeding, and listlessness. Infection can lead to meningitis and septicemia. Infants with low birth weight or immune system problems have an increased risk of infection. Despite the rare occurrence of this illness, its severity should not be misjudged as it can result in long-term complications or death. The CDC recommends that any infant with C. sakazakii infection be brought to a physician for treatment.
Further, the CDC recommends breastfeeding over formula use. However, when breastfeeding is not feasible, the CDC and FDA provide the following recommendations for infant formula use:
- Wash hands and all feeding utensils with soap and water before beginning preparations.
- Water used in infant formula should be boiled first and then cooled quickly to serve to the infant.
- Only prepare enough formula for one feeding and discard any leftovers.
- Read “use by” date, storage information and preparation methods very carefully.
Resources:
Reporting a problem to the FDA:
http://www.fda.gov/Safety/ReportaProblem/ucm2005658.htm
FAQs and helpful hints from FDA on infant formula:
http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048694.htm