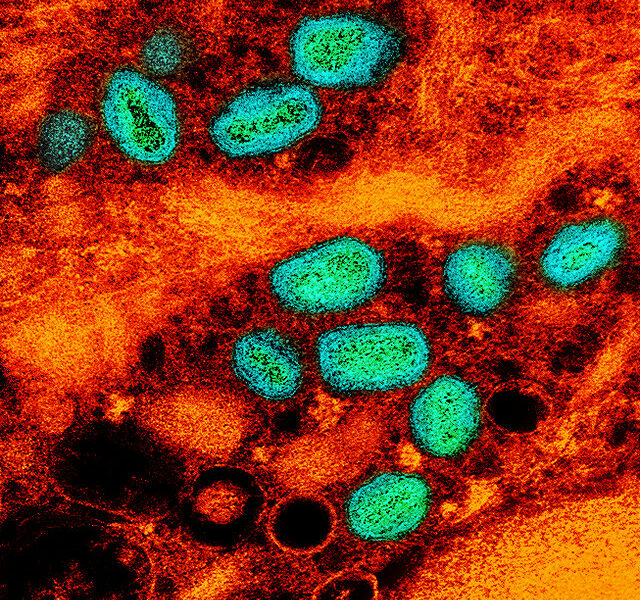
Since March of this year, New Jersey based Princeton University has been dealing with an onslaught of severe bacterial meningitis cases. Last week, the eighth case was reported when a female student was hospitalized after presenting symptoms of meningococcal disease. The first seven cases were all caused by the bacterium Neisseria meningitidis. Lab results for this most recent case are pending.
The school is suffering from an outbreak of a particular type of meningitis: Serotype B, for which no vaccine currently exists in the United States. At first, in an effort to combat the disease, school officials offered preventative antibiotics and encouraged behaviors that minimize the spread of the disease. However, as the infection spreads, the Ivy League may turn to different measures.
According to New Jersey law, all students entering a four-year institution must be immunized against meningococcal disease. However, the vaccine currently licensed in the US only covers certain types of meningococcal disease, including A, C, Y, and W, but not type B.
To protect students against this type of meningitis, the University is now preparing to provide a vaccine not yet licensed for use in the United States. The unlicensed vaccine, marketed as Bexsero and manufactured by Swiss drug maker Novartis, was approved in Europe in January and in Australia in August. Additionally, the FDA also considers the vaccine safe and has allowed Princeton to use it under an Investigational New Drug Application; a term used to describe a medication or a vaccine that is not yet approved in the US but may be used under certain circumstances.
More than 8,000 infants, children, adolescents, and adults were safely immunized in the drug trials that led to the vaccine’s approval in the European Union and Australia. The most common reported side effects were pain, tenderness, swelling, and hardness of the skin at the site of the vaccine injection. Other side effects included nausea, feeling run-down, and headaches. As with any vaccine, there is a very small risk that a more serious problem can occur, such as a severe allergic reaction.
Pending approval by the Centers for Disease Control (CDC), the University will provide the vaccine for all undergraduate students as well as any graduate students living in dormitories. Additionally, individuals with specific medical conditions such as immune disorders may be evaluated for vaccination. Princeton University will cover the cost of the vaccine for all eligible individuals.
In a similar but as of yet, unrelated outbreak, three students form the University of California Santa Barbara (UCSB) are also being treated for meningitis B. Although it is the same strain as the one affecting Princeton University, public health officials have not discovered a connection between the two outbreaks, and don’t expect to.
“This is not unexpected, as cases of meningococcal disease can occur sporadically in college settings since this population has an increased risk,” said the Santa Barbara County Department of Public Health in a recent press release.
UCSB and the department will closely monitor the outbreak as they also explore the possibility of meningitis type B vaccination.
College students, particularly those living in residence halls, are at an increased risk for meningococcal disease. Meningitis is spread from person to person through saliva and respiratory secretions during close and lengthy contact. Coughing, kissing, and sharing food, drinks, or cigarettes all promote the spread of the bacteria. To prevent the spread of the illness, students are encouraged to always cover their mouths and noses when sneezing, practice hand hygiene, and avoid sharing utensils, water bottles and other items contaminated by saliva or respiratory secretions.